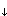Hexachlorobenzene
A concentration of 100 µg hexachlorobenzene (HCB) per kg wet weight has been measured in fish that live and in the sediment/water interface. The average fat content of the fish is about 5 % (i.e. 0.05 kg lipid per kg wet weight). The aqueous concentration of HCB in water was determined to be 0.1 ng L-1, in the sediment (foc = 0.05 kgockg-1 in dry sediment) a concentration of 2 µg kg-1 sediment (dry) was analysed.
Estimate (roughly) which concentration one would expect in the fish (in µg per kg wet weight) if they were in equilibrium with
a) the water phase or
b) the sediment phase.
What assumptions do you make?
Use the following partition constants:
log Klipid/water = 5.8 (Lwater/Llipid)
log Koc/water = 5.3 (Lwater/kgoc)
Answer:
a) 100 µg per kg wet weight correspond to 2·106 ng per kg lipid. In equilibrium with water one would expect 6.3·104 ng/kglipid if HCB only partitions into fish lipids.
On basis of the total wet weight of the fish one would expect a concentration of 3.15 µg/kgwet weight instead of the 100 µg/kgwet weight that have been measured.
b) The Koc is 2 ·105 Lwater/kgoc. The sediment/water partition constant thus is 1·104 Lwater/kgsediment because only 5% of the sediment is organic carbon. The equilibrium concentration in the pore water then was about 2·10-4 µg /Lwater oder 0.2 ng/Lwater based on the measured 2 µg kg-1 in wet sediment. In equilibrium with the sediment one would expect 1.26·105 ng/kglipid if HCB only partitions into the fish lipids.
Conclusion: The fish cannot have been contaminated from either the water or the sediment phase. Most likely they have been contaminated via the food chain and the up-take kinetics with the food are so much faster than the clearance kinetic via the water phase that no equilibrium with water is achieved.
Download this page as a pdf