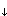Chlorobenzene
a) A small amount of sorbent (volume 0.66 µL) is equilibrated with a water sample of 5 ml. In the subsequent analysis one 0.2 µg monochlorobenzene in the sorbent. The partition constant at room temperature is: log K = 2.89 (with K = Cchlorobenzene in sorbent (g/m3) / Cchlorobenzene in water (g/m3)). What has been the concentration monochlorobenzene in the original water sample? How much (in %) of the total amount of monochlorobenzene was eventually sorbed in the sorbent?
b) With the same water sample and the same sorbent as in a): What would have been the influence if there had been an air phase of 7 ml present during the equilibration of sorbent with the water. Which amount of monochlorobenzene would you expect to find in the sorbent in this case? The air-water partition constant is: KH = 0.15 (with K = Cchlorobenzene in air (g/m3) / Cchlorobenzene in water (g/m3) at room temperature.
Answer:
a) 9.3 % must have been in the sorbent. The total amount of chlorobenzene must have been 2.15 µg. Hence, the original concentration in water must have been 0.43 mg/L.
b) In this case 16 % of the monochlorobenzene would have been in air and only 7.8 % in the sorbent.
Download this page as a pdf