Raining out
Because of the increasing contamination of the atmosphere by organic pollutants, there is also a growing concern about the quality of rainwater. In this context, it is interesting to know how well a given compound is scavenged from the atmosphere by rainfall. Although for a quantitative description of this process, more sophisticated models are required, some simple equilibrium calculations are quite helpful.
Assume that PER and MTBE (see below) are present in the atmosphere. Consider now a drop of water (volume ~ 0.1 mL) in a volume of 100 L of air [corresponds roughly to the air-water ratio of a cloud]. Calculate the fraction of the total amount of each compound present in the water drop at 25°C assuming equilibrium between the two phases.
Answer:
In general, we can use sheet A from Mehr-Phasensystem.xls to answer this question:
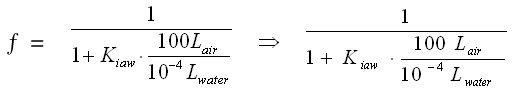
PER: the fraction in the water phase is almost zero (8.3E-7). This compound will not be removed significantly from the atmosphere by rainfall.
MTBE: the fraction in the water phase is 4.2E-5 = 0.0042%. This is also very small. A lot of rain will be needed to remove MTBE from the atmosphere.
Download this page as a pdf