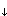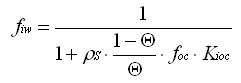What fraction of atrazine is present in dissolved form?
Atrazine is still one of the most widely used herbicides worldwide. Estimate the fraction of total atrazine present in truly dissolved form (a) in lake water exhibiting 2 mg particulate organic carbon (POC) suspended per L water, (b) in marsh water containing 100 mg solids L-1, if the solid's organic carbon content is 20%, and (c) in an aquifer exhibiting a porosity of 0.2 by volume, a density of the minerals of 2.5 kg L-1, and an organic carbon content of 0.5%. Assume that partitioning to organic matter is the major sorption mechanism.
 |
||
| Use the following sorption constant for atrazine | ||
If you are confused because you do not understand what to do with the organic carbon content and how to deal with phases that are not quantified by volume then it is high time to read chapter 3 in the textbook.
Question: What practical relevance do the results have?
Help: You can use spreadsheet C for all calculations: The total volume that you choose does not matter for the results. For simplicity take 1 litre and choose all other volumes according to the information given in the question.
If you want to do the calculation by hand use ...
| for (a) and (b): | 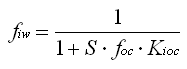 |
S foc = [POC] (particular organic carbon), |
| for (c): |
|
| Proceed | |