Detailed description of FD
Similar to UV detectors, fluorescence detectors use monochromatic light to irradiate a flow cell that is connected to the HPLC. Instead of measuring absorption of the eluting compounds, fluorescence detectors measure the intensity of light of a given emission wavelength that is emitted by the analyte molecule when it relaxes from its excited state - to which the molecule was transferred by light absorption - back to its ground state. The emitted light, which is of longer wavelength than the excitation wavelength, is referred to as fluorescence light. It is emitted in all directions and typically, but not necessarily, detected at a 90° angle to the excitation beam. Many detectors allow the simultaneous measurement of both the excitation and the emission (or monitoring) wavelength of a fluorescent molecule.
Compared to UV-visible absorption detectors, fluorescence detectors are highly selective because not all organic molecules that absorb light also fluoresce. Molecules that do, may principally be quantified by either UV-visible or by fluorescence detectors. However, the latter are often by a factor of 1000 more sensitive than UV-visible absorption detectors.
One complication that may arise in fluorescence detectors is that the fluorescence light emitted by the analyte may be absorbed in solution, either by dissolved ions, different chemicals, or by other analyte molecules. This process, which is referred to as self-absorption, typically results in non-linear responses of the detector at lower analyte concentrations than for UV-visible absorbance detectors.
Among the organic pollutants that are often quantified using fluorescence detection are polycyclic aromatic hydrocarbons (PAHs), such as:
| naphthalene | |
| phenanthrene | 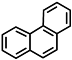 |
| or pyrene | 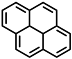 |




 Detailled description of FD
Detailled description of FD

