Detailed description of UV-vis-AD
Ultraviolet-visible (UV-vis) light absorption detectors typically contain a deuterium lamp to generate UV-light (i.e., 200 - 400 nm) and a tungsten filament lamp to generate visible to near infrared light (400 - 1600 nm). The light emitted from the lamps is passed through a monochromator − either a monochromatic filter or a diffraction grating − to obtain light of only one desired wavelength, so-called monochromatic light. Its wavelength is dependent on the position of the monochromator relative to the light source.
The beam of monochromatic light is then split into a measurement and a reference beam. The intensity of the measurement beam I(![]() ) is determined after it passed through a flow cell that is connected to the outlet of the HPLC column. Contrarily, the intensity of the reference beam I0(
) is determined after it passed through a flow cell that is connected to the outlet of the HPLC column. Contrarily, the intensity of the reference beam I0(![]() ) is directly measured (i.e., the reference beam does not pass through the flow cell). If an organic analyte elutes from the column and if that analyte absorbs light at the wavelength of the monochromatic light, I(
) is directly measured (i.e., the reference beam does not pass through the flow cell). If an organic analyte elutes from the column and if that analyte absorbs light at the wavelength of the monochromatic light, I(![]() ) is attenuated while I0(
) is attenuated while I0(![]() ) is not affected.
) is not affected.
For a compound i and a measurement wavelength ![]() , absorbance Ai (
, absorbance Ai (![]() ) (unitless) is defined as:
) (unitless) is defined as:
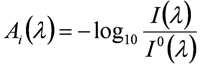
Ai (![]() ) is proportional to the total number of analyte molecules in the measurement beam, and hence to the concentration. This linear relationship is described by the Lambert-Beer law:
) is proportional to the total number of analyte molecules in the measurement beam, and hence to the concentration. This linear relationship is described by the Lambert-Beer law:
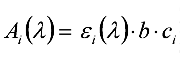
where εi(
In other words, UV-vis detectors measure the fraction of monochromatic light that passes through a sample. Changes in the intensity of the measurement beam relative to the reference beam are detected and reported as a chromatographic peak.




 Detailed description of UV-vis-AD
Detailed description of UV-vis-AD 

