General description of ECD
Operating principle:
Eluting analytes scavenge electrons emitted by a radioactive source in the detector; the resulting drop in electric current generates a signal.
sensitivity: |
|
increasing electro-negativity of analyte (i.e., electron scavenging efficiency of the analyte) |
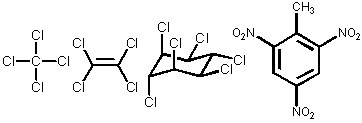
| well suited to detect analytes that contain nitro-, carbonyl-, and halogen groups | ||
detectable by ECD: |
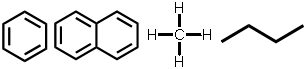
NOT suitable for detection by ECD: |
 |
 |
|
|




 General description of ECD
General description of ECD

