Rules for partitioning of various compounds (3/5)
These data reveal another general finding that is noteworthy: water is the only phase in which the cohesive energy is so strong that the cavity energy is −slightly− larger than the van der Waals energy that a molecule i can gain from its interactions. Consequently, for water the following rule holds:
Rule 9: |
Examples are shown in Figure 5 below.
Note that due to the dominance of the cavity energy over the van der Waals energy, alkanes and other apolar compounds prefer to stay in the gas phase rather than in water (see Figure 5)
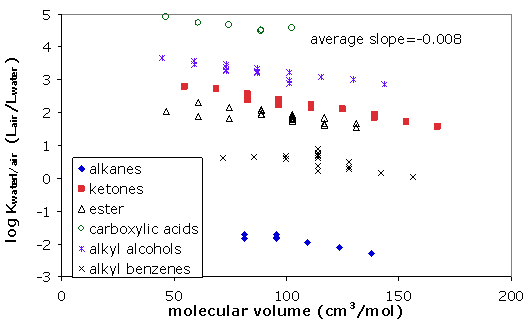
Figure 5: Water-air sorption coefficients plotted for various compound classes versus the respective molar volumes of the chemicals.
Download this page as a pdf




 Page 3/5
Page 3/5
