Intermolecular interactions in every day life
Many things in our every day life are affected by the same intermolecular interactions that also govern the partitioning of molecules. For example, (organized) organic phases would not exist if there were no van der Waals and Hydrogen bond forces that would keep them together. Here, we have picked some special effects whose working principles may not be obvious immediately.
We have pointed out already that van der Waals interactions occur between all types of molecules and that they always are attractive. But so far we have only looked at single molecules. Forces between them are very small and cannot directly be related to our every-day life experience. Extrapolating these interactions to macroscopic dimensions (e.g. the area of a hand) yields amazingly high forces. These intermolecular forces are responsible for many sometimes intriguing phenomena.
How does plastic wrap work? This question was answered in the german TV-show "Kopfball": |
 |
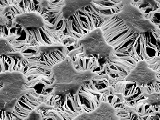
How can a Gecko climb a wall? Have a look at our explanation or at this article: |
 |
Lotus effect What are self-cleaning surfaces? |
 |
Gore-Tex How do membranes work that are waterproof and at the same time breathable? |
 |
Club moss (Lycopodium) One of the entertaining effects of intermolecular interactions: |
 |
Magic sand Some more entertaining with hydrophobic sand: |
 |
Wet sand in a pail And another example for how powerful van der Waals and Hydrogen-bond forces can be. |
 |
Download this page as a pdf


 Intermolecular interactions in every day life
Intermolecular interactions in every day life