How do membranes work that are waterproof and at the same time breathable?
If you ask at the sports outfitters how a water proof but breathable membrane works the you will most likely get the following answer: the membrane has pores that are so small water droplets (rain) cannot pass while water vapor (i.e. single water molecules) can. This explanation sounds plausible and can also be found on wikipedia.
Have a look: http://en.wikipedia.org/wiki/Gore-Tex
While this answer is not incorrect, it is still far from the truth.
Just making tiny pores into any thin membrane does not at all create the desired effect. You may have witnessed that water creeps upwards in tiny (glass) capillaries even against gravity. On huge scales this happens in our soils and this is quite fortunate because otherwise much of the global land surface would be too dry for agriculture. This upward movement is triggered by the strong attractive forces (especially H-bonds) between the water and surface materials such as glass and quartz. And the smaller the capillaries or pores, the more contact the water can make with these surfaces and the higher it can rise due to these surface forces.
Cotton (= cellulose) carries many OH-groups and is therefore also a material with which water can form strong H-bonds:
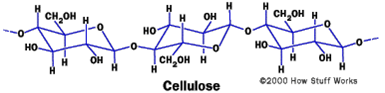
For that reason cellulose can take up water until the 25fold of its own weight. Hence, a cotton fabric with very tine pores would just have the opposite effect of what is desired here: water would eagerly creep through all these tiny pores that act as capillaries to the inside of the fabric searching for more and more possibilities to engage in strong H-bonding with the fabric.
However, nobody would pay the price of a Gore-TexTM jacket for such a thing. Hence Mr. Gore, the inventor of this fabric, must have been a little smarter than our sports fitters tries to tell us.
The trick is to use a material, e.g TeflonTM, that has little attractive interactions with water. TeflonTM is not water repellent as you have seen already (link) but it forms no H-bonds with water and the van-der-Waals interactions that TeflonTM offers to the water molecules are smaller than what the water molecules experience between each other. Hence, water molecules in a rain drop rather stay in contact to each other than maximizing the contact to this kind of surface. In such a case a capillary shows an effect opposite to that of a glass capillary: the smaller the pore or capillary the more energy it takes to force a water droplet through this pore because more of the water molecules in the droplet have to be forced out of their own environment, which provides them with lots of H-bond and van-der-Waals interaction energy, into contact with a TeflonTM surface where they gain much less interaction energy. The pressure that it takes to force water through such a membrane (and which may be exerted for example when wearing a back pack) is thus a function of the material of the membrane and the size of the pores and this pressure is used as a quality criterium for outdoor garment.
Note: salts that we excrete via our skin deteriorate the function of the membrane when they get into the pores because of their high attractivity for water.
Download this page as a pdf




 Gore-Tex
Gore-Tex