Potential sources of analyte losses
CAUTION! Large systematic errors may arise during
sampling and sample storage. Two major causes:
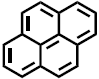
1. Sorption of analyte to sampling |
e.g., pyrene high plastic-water partition constant Kplastic/water |
plastic: NO glass: YES
|
| Water molecules (55.4 mol/L) out-compete the organic compound for sorption sites on the polar silica wall of the glass. This is the reason why glass bottles are almost always better than plastic bottles. However, inorganic cations (e.g., heavy metals, ...) may sorb strongly to the silica glass surface. Therefore, for environmental trace analysis of inorganics, plastic bottles are typically preferred. | ||
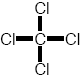
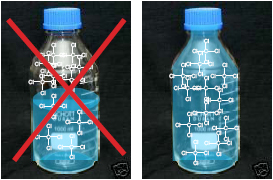
| 2. Volatilization of analyte molecules during sampling or sample storage |
e.g., tetra- chloromethane high air-water partitioning constant Kair/water |
headspace: NO full bottle: YES
|
| |
||
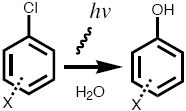
| 3. Losses of analyte to to photoinduced chemical transformation (will be later covered in the lecture; chemical transformation) |
e.g., substituted chlorobenzenes (photolabile) |
clear glass: NO amber glass: YES
|
... and many more potential processes, e.g.:
4. Loss due to microbial degradation of the analyte during storage.
Microbial activity is often reduced/eliminated by adding a bacteriocide (e.g., Sodium azide) or by adding acid to lower the pH. While the latter inactivates microorganisms, it may also facilitate abiotic degradation of certain organic pollutants (see acid catalyzed reactions in the "Transformation reactions" section).
It is useful to refer to the literature prior to a sampling event and adapt the sampling strategy to the targeted analyte (i.e., its partitioning characteristics and chemical stability) (e.g., does the chemical sorb to plastic, does the chemical have a high air-water partition constant, is the chemical photolabile, ... ?)

 Potential sources of analyte losses
Potential sources of analyte losses