Transfer accross phase boundaries
Phase boundaries always consist of two adjacent laminar layers. Transport through these layers can only occur by molecular diffusion and this typically is the rate-limiting step in the exchange between phases.
Example: Air-water exchange

Air-water exchange:
-
- Typically, due to turbulent mixing, there are no substantial concentration gradients within the bulk air and water phase in the vicinity of the interface. Gradients only exist directly at the interface and between the two laminar films.
=> phase transfer is a diffusion process that can be treated as stationary within short time intervals.
- - the driving force is not a concentration gradient but a partition disequilibrium: (cwater - cair/Kair/water) with Kair/water = equilibrium partition constant of the considered compound between air and water
-
- the diffusive phase transfer depends on the thickness of the laminar films (= diffusion path length), the diffusion coefficients in both media and the equilibrium partition constant.
With the assumption that there is a partition equilibrium directly at the interface between the two adjacent laminar layers Kaw= c*i air / c*i water , one can derive the following formula for the rate of transfer (for details on the derivation of this equation see our textbook)
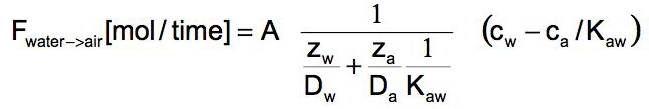
with z = thickness of the laminar layer (typically in the range of < 1 mm depending on wind and water velocity), A = interfacial area, Kaw= air/water equilibrium constant.
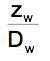 and
and  can be interpreted as transport resistancies.
can be interpreted as transport resistancies.
Similar to an electric current, the flux is inverse proportional to the sum of the two resistances that are in series.
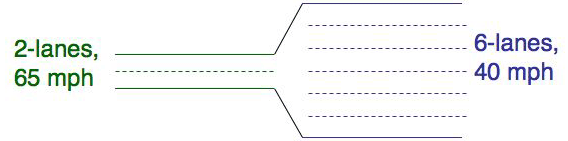
It is plausible that the transport resistancies depend on the diffusion coefficients D and the thickness of the laminar layers z, but it is not directly intuitive why the relative resistance in both laminar layers also depends on Kaw. There is an analogy from traffic where cars stand for the molecules that can illustrate this: Imagine the situation shown in the figure below. The stretch of road with two lanes and a rate limit of 65 mph is comparable to the laminar air film: i.e. the capacity (=number of cars that can drive on this road at any time) is small but the possible speed of each car (=diffusivity) is high. The laminar water layer rather resembles the 6-lane road where the maximal capacity to hold cars is larger but the maximum speed is small. This explains why the relative capacity of the two laminar films also matters for the transfer resistance. And for molecules this relative capacity is nothing else than the partition constant K. More information on transfer resistances can be found in our text book and for organisms and passive samplers in Bayen et al. (2009).
Note: Although D is larger in air than in water for any compound and although the laminar layer is always thinner on the air-side than on the water side, there are still situations where the air-side controls the overall transport kinetics (i.e., resistance in air >> resistance in water): this happens when Kair/water is very small (<<1).
Download this page as a pdf

