9) Explain and estimate saturated vapor pressure
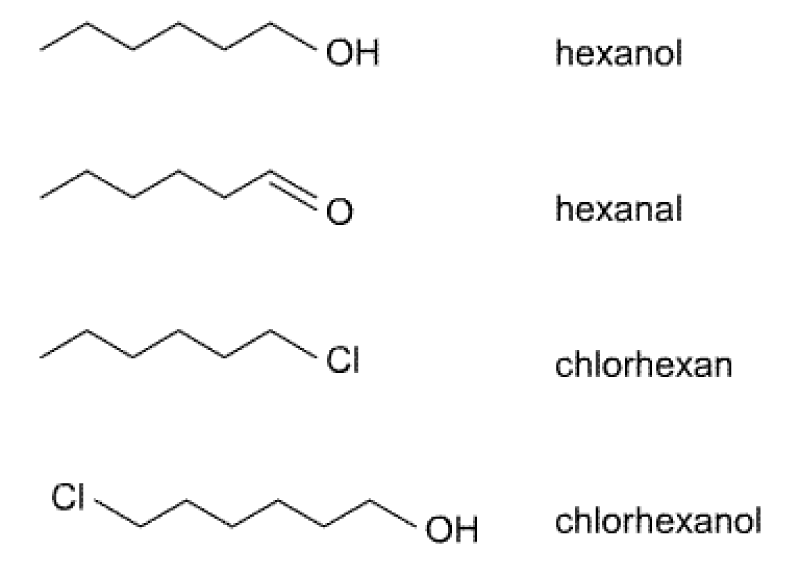
At 25°C these liquid compounds have the given saturation vapour pressures:
n-Hexane
20 000 Pa
Hexane-1-ol
110 Pa
1-Chlorhexane
1260 Pa
-
What is the reason for those large differences in saturation vapour pressure?
-
Could you calculate the saturation vapour pressure for 1-chlorhexanol from the given values?
Please provide a short explaination for your answer. -
Inbetween which of the values above would you rank the saturation vapour pressure of hexanal?
You can check your answer in the next lesson.
Download this page as a pdf




 9) Explain saturated vapor pressure
9) Explain saturated vapor pressure
