4) Fuel accident
Question:
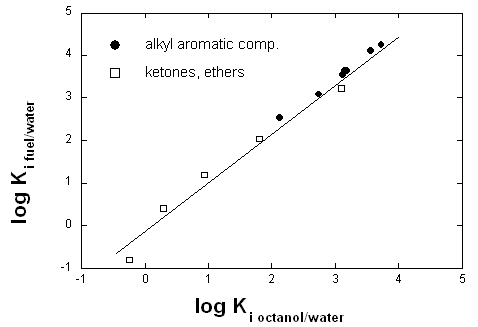
Fuel was spilled in a traffic accident. The local water supplier is concerned that this fuel may have reached the ground water table. In order to set up a worst-case scenario, you are asked to estimate the fuel /water partition constants of various phenols and anilines, which are known to occur in trace amounts in the fuel. In the literature you can only find the fuel/water partition constants of alkylaromatic compounds, ethers, and ketones (see figure). Can you use this knowledge to estimate the fuel/water partition constants of phenols and anilines whose octanol/water partition coefficients are known? (The fuel is a mixture of alkanes and alkylaromatic compounds and Methyl-tert-butylether (MTBE)).
Answer:
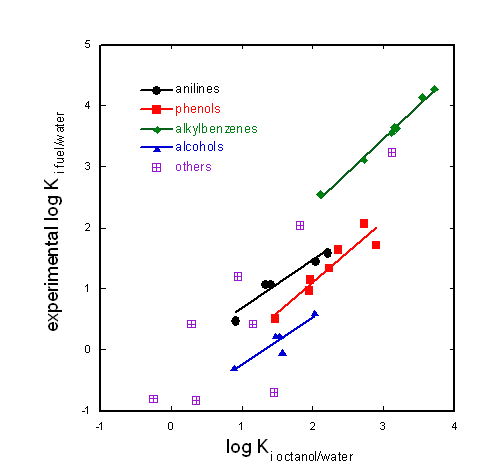
No you cannot. Fuel is a mixture of apolar and monopolar (H-bond accepting) solvents. Octanol is bipolar and can therefore not mimick fuel for H-bond donor compounds that are sensitive to just this property. In the graphic only H-bond accepting chemicals are shown. They are not sensitive to this difference between octanol and fuel. But phenols and anilines are H-bond donating compounds and therefore sensitive to this difference (see figure below).
Download this page as a pdf






