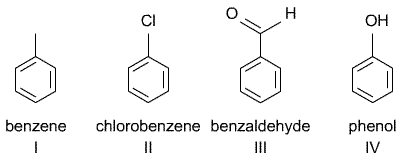
12) Tendency to distribute
Question:
Rank the four compounds (I − IV) indicated below in the order of increasing tendency to distribute from (a) air into hexadecane (mimicking an apolar environment), (b) from air to olive oil, and (c) from air to water
These are the respective volumina for the compounds:
benzene 0.716
chlorobenzene 0.839
benzaldehyde 0.873
phenol 0.775
| proceed to answer | |