Box 10: Principles of filtration of sorbing compounds
Everybody is familiar with filters that retain particles with larger diameters than the pore sizes in the filter. Such filters can clog after extended use.
The same principle could also be used for removing pollutant molecules from water or air by using porous membranes. However this method is neither very selective to certain types of pollutants nor does it allow a high throughput of sample volume in short time with reasonable technical and financial effort. Hence, the removal of pollutants from air or water (e.g. the extraction of analytes from environmental samples or cleaning of exhaust gas or waste water) often relies on sorbent materials that hold back the target compounds by intermolecular interactions (van-der Waals, H-bond, electrostatic interactions). Hence, retention of pollutants in a filter is a function of their partition equilibrium between the mobile phase and the sorbent. This is very similar to retention in a chromatographic system (see Box 13 in script ).
While pollutants are introduced in short pulses to chromatograhic systems, pollutants typically enter the filters over the entire time when contaminated air or water is flushed through the filter. Just like in chromatography this sorption is neither complete nor irreversible so that it is only a question of time until the first pollutant molecules can be found in the filter outflow. This concentration will continue to rise until the concentration in the outflow is identical to the concentration in the inflow, i.e. there remains no net sorption because the filter has reached complete partition equilibrium with the analyte in the air or water that was flushed through it. The curve that describes the concentration of the pollutant in the outflow is the so-called breakthrough curve, BTC (see Figure below). The retention time or retention volume can be calculated as shown in the script.
The form of the BTC (i.e. how steep or shallow the slope is) depends on the effective dispersion/diffusion of the compound in the filter and on the retention factor. The effective diffusion coefficient of a compound may be estimated theoretically. The effective dispersion coefficient mainly depends on the filter characteristics (e.g. pore size distribution) and is best determined from a calibration experiment. With this information at hand the BTC of a compound can be predicted using the appropriate solution of the convective/dispersive transport equation. In most cases the water or air quality criteria will be such that only a small percent of the incoming concentration is acceptable in the outflow. The time for which a filter can be used or, better, the volume of air or water that can be cleaned with a filter before it has to be replaced can be estimated from the breakthrough curve.
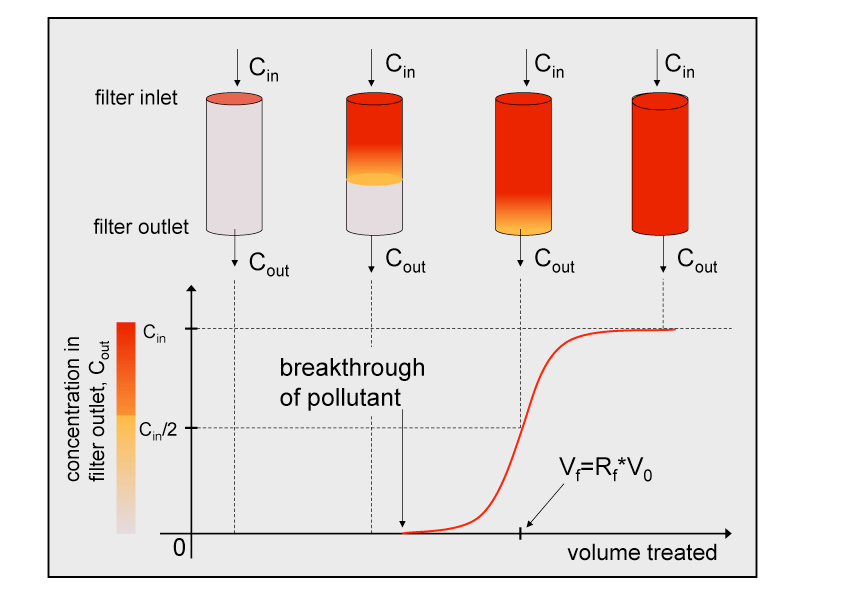
Figure Example of a breakthrough curve through a filter for a homogenous input of a compound with the concentration Cin.
Download this page as a pdf


 Box 10 Filtration
Box 10 Filtration
