Equilibrium partitioning in quantitative terms
Let us now assume that, in a first step, we have a chemical i that has been added in a small amount to a system with two phases 1 and 2. At equilibrium (indicated by the *), let the concentration of i in phase 1 be 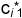 and the concentration of i in phase 2 be
and the concentration of i in phase 2 be 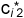 . Of course these concentrations depend on the relative interaction energy of compound i with the phase molecules of 1 and 2 in the two phases.
. Of course these concentrations depend on the relative interaction energy of compound i with the phase molecules of 1 and 2 in the two phases.
Assume further that, in a second step, we add the same amount of i to the system as in step 1. What will be the new equilibrium concentrations of i in the two phases? Clearly, the new equilibrium concentrations will be 2 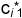 and 2
and 2 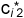 . Why? Because the molecules i added in the second step encounter the same interactions and therefore partition in the same way as the molecules i added to the system in the first step. In other words, the RATIO of the equilibrium concentrations of i in the system will stay constant. This ratio only depends on the molecular interactions of i with the phase molecules of phases 1 and 2. The ratio of
. Why? Because the molecules i added in the second step encounter the same interactions and therefore partition in the same way as the molecules i added to the system in the first step. In other words, the RATIO of the equilibrium concentrations of i in the system will stay constant. This ratio only depends on the molecular interactions of i with the phase molecules of phases 1 and 2. The ratio of 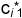 to
to 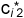 is called the partition constant Ki 12 .
is called the partition constant Ki 12 .
Ki 12 ![]()
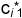 /
/ 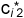 (1)
(1)
Remember this definition of the partition constant: the first phase that is mentioned in the subscript of K is the one whose equilibrium concentration is given in the numerator of the equilibrium concentration ratio. We will use this definition throughout this text. Be aware that other definitions may be used elsewhere.
In summary: The equilibrium concentrations of compound i in two different phases 1 and 2 will depend on the absolute amount of i added to the system but the CONCENTRATION-RATIO at equilibrium will be the same.
Exceptions: There are situations where the equilibrium partition ratio is not constant but a function of concentration. It directly follows from the above description that this must happen when molecules i encounter interactions at low concentrations different from those at high concentrations. This is the case when the sorbing phase is heterogenous or when there are so many molecules i in the phase that these molecules start to interact with themselves. More details on this will be given later.
Download this page as a pdf




 EP in quantitative terms
EP in quantitative terms