Analyte mixtures and separation - 3
Instead of extraction with different solvents (or different sorbent phases), use of:
- only one solvent (or sorbent phase) as a 'stationary phase'
- analytes introduced into a 'mobile phase'
- analyte partitioning between mobile and stationary phase
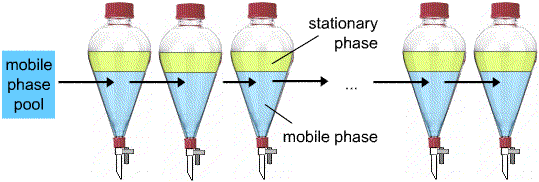
Slight differences in Ki, stationary phase/mobile phase between analytes result in their separation upon successive re-equilibration between the two phases.
Repeated equilibration of analyte molecules between a stationary phase and a mobile phase is called CHROMATOGRAPHY.