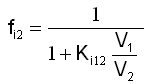In environmental chemistry we want to know:
|
|
|
All this information can be calculated based on the respective partition constant and some additional information: In the script the following formula is derived for the fraction of chemical i that resides in phase 2 in a two-phase system in equilibrium. V1 and V2 are the volumes of the two phases and Ki 12 is the respective partition constant expressed in volumetric concentrations. |
|
Download this page as a pdf

 In environmental chemistry we want to know:
In environmental chemistry we want to know: