Who is right - the power company or the citizens?
In Switzerland there was a dispute between an electric power company and a local organisation of citizens about whether the high-voltage power lines should run above the ground (the option favoured by the company) or below ground. The company argued against below ground lines as these need to be isolated with a gas, SF6 , which is one of strongest known green-house gases. The citizen´s answer to that was that this gas is 5 times denser (heavier) than air and can therefore never reach the upper atmosphere (see SF6 Problem.pdf (in German)).
Can you decide who is correct?
Answer: According to what you have learned so far, you should argue that gases will eventually evenly fill out the complete available space. Hence, the gas should reach the upper atmosphere. But what about gravity effects? On the one hand, we have argued that the partitioning of molecules is a result of intermolecular interactions and the thermal energy of the molecules. On the other hand, all things that possess a significant mass should also be subject of gravitational forces, which we have neglected so far. In fact, gravity indeed affects the distribution. However, this effect is so small that it may be neglected in most cases.
This is how we assess the effect of gravity:
Eq. 2 describes the effect of various forces on the partitioning of molecules:
![]() 12 Gi = -R T ln Ki 12 (2)
12 Gi = -R T ln Ki 12 (2)
In an ideal (i.e., dilute) gas phase, the intermolecular forces between the molecules are almost zero. This means that there is a homogenous distribution of all gas molecules in space. Now, in order to account for gravitational effects, we have to calculate the work that is needed to bring a molecule i from altitude z1 to altitude z2. This work is equivalent to the free energy of phase partitioning, ![]() 12 Gi , of this chemical i in the gas phase between these two altitudes. This gravitational work is given by mi g (z2 - z1) where m i is the molar mass and g is the gravitational acceleration. K i 12 in eq. 2 corresponds to the equilibrium concentration ratio c * i1 in height z1 and c * i2 in height z2. Hence, we can rewrite eq. (2) as:
12 Gi , of this chemical i in the gas phase between these two altitudes. This gravitational work is given by mi g (z2 - z1) where m i is the molar mass and g is the gravitational acceleration. K i 12 in eq. 2 corresponds to the equilibrium concentration ratio c * i1 in height z1 and c * i2 in height z2. Hence, we can rewrite eq. (2) as:
| (3) |
When we insert the molar masses of the most abundant atmospheric gases (oxygen and nitrogen) then this equation becomes nothing else than the barometric distribution law. This law describes the decrease in density of our atmosphere with increasing altitude. This equation also shows that the effect of gravitation on partitioning of typical pollutant molecules (with a molar mass smaller than 500 g/mol) only becomes noticeable when looking at very large differences in altitude. (See http://www.falstad.com/gas/ for an animation of the effect of temperature and gravity on the distribution of a gas with height.)
Applied to our original problem we can now conclude that SF6 will indeed partition into the atmosphere. Its equilibrium concentration will decrease exponentially with height. Using the molar mass of SF6 we can calculate that the equilibrium concentration 10 km above ground is 400 times smaller than on the ground (ignoring the influence of any temperature difference). Hence, the power company was principally right. However, it remains open whether the amount of SF6 that could enter the atmosphere would actually matter to the climate or not.
Download this page as a pdf

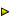



 Answer
Answer